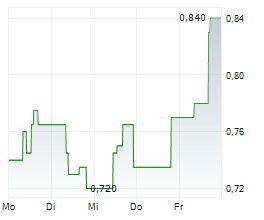
WASHINGTON (dpa-AFX) – Curis, Inc., (CRIS) announced the FDA has removed the partial clinical hold on the TakeAim Leukemia Phase 1/2 study of emavusertib. On April 4, 2022, the FDA placed a partial clinical hold on the study. Also, the recommended phase 2 dose for emavusertib as a monotherapy has…Read More
Curis FDA Removes Partial Clinical Hold On TakeAim Leukemia Study



:quality(70)/cloudfront-us-east-1.images.arcpublishing.com/cmg/S22V2GGPN5CD3CG7D55626CSR4.jpg)